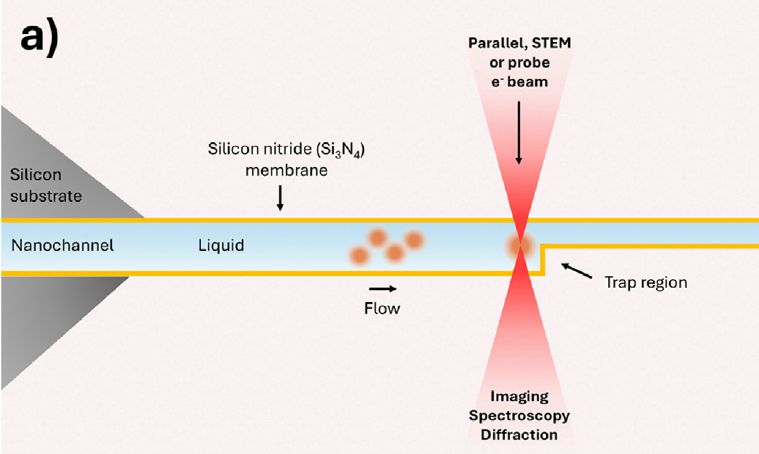
Our latest results with the Nano Channel Chips
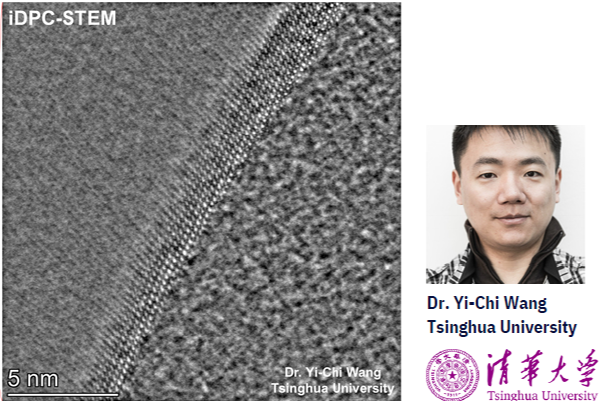
Best atomic resolution image...ever?
At a demo with researchers at Tsinghua University, we not only got the first ever iDPC-STEM images taken, but also the most impressive atomic resolution images we've ever had taken in our Nano Channel Chips.

App-note: Flow and Diffusion Dynamics of Rhodamine B fluorescence dye in Nano Channels
An experiment to examine flow and diffusion dynamics inside nano channels of Insight Chips’ Nano Channel Chip using fluorescence microscopy.

Cathodoluminescence in liquid cell TEM?!
Attolight's Mönch and Insight Chips' liquid cells are now tested together, and are finally verified to be compatible!
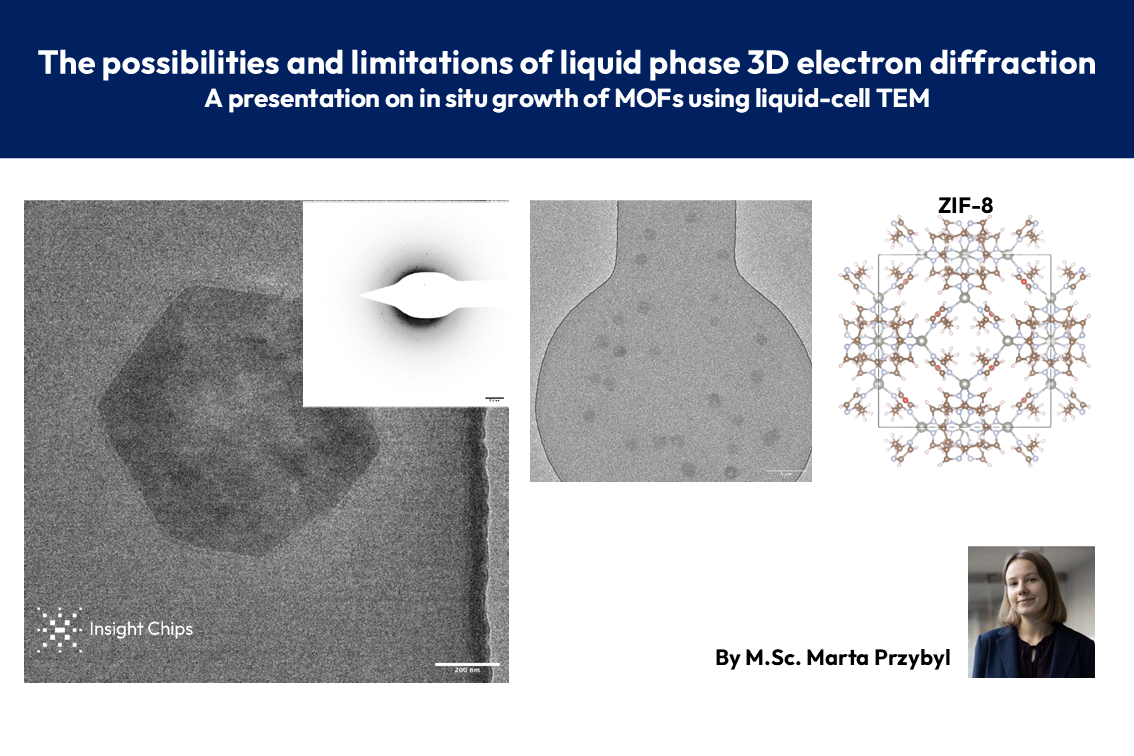
In-situ growth of MOFs in liquid TEM - Online presentation
📢 Exciting results for anyone interested in MOF research and liquid-cell TEM!
CaCO3 growth in liquid by mixing of CaCl2 and Na2CO3
Precipitation of CaCO3 can be observed in the Nano Channel Chips by utilizing the mixing feature. When CaCl2 and Na2CO3 come into contact in the chip, crystal formation can be observed, in this case in liquid of 60 nm and <5 nm!
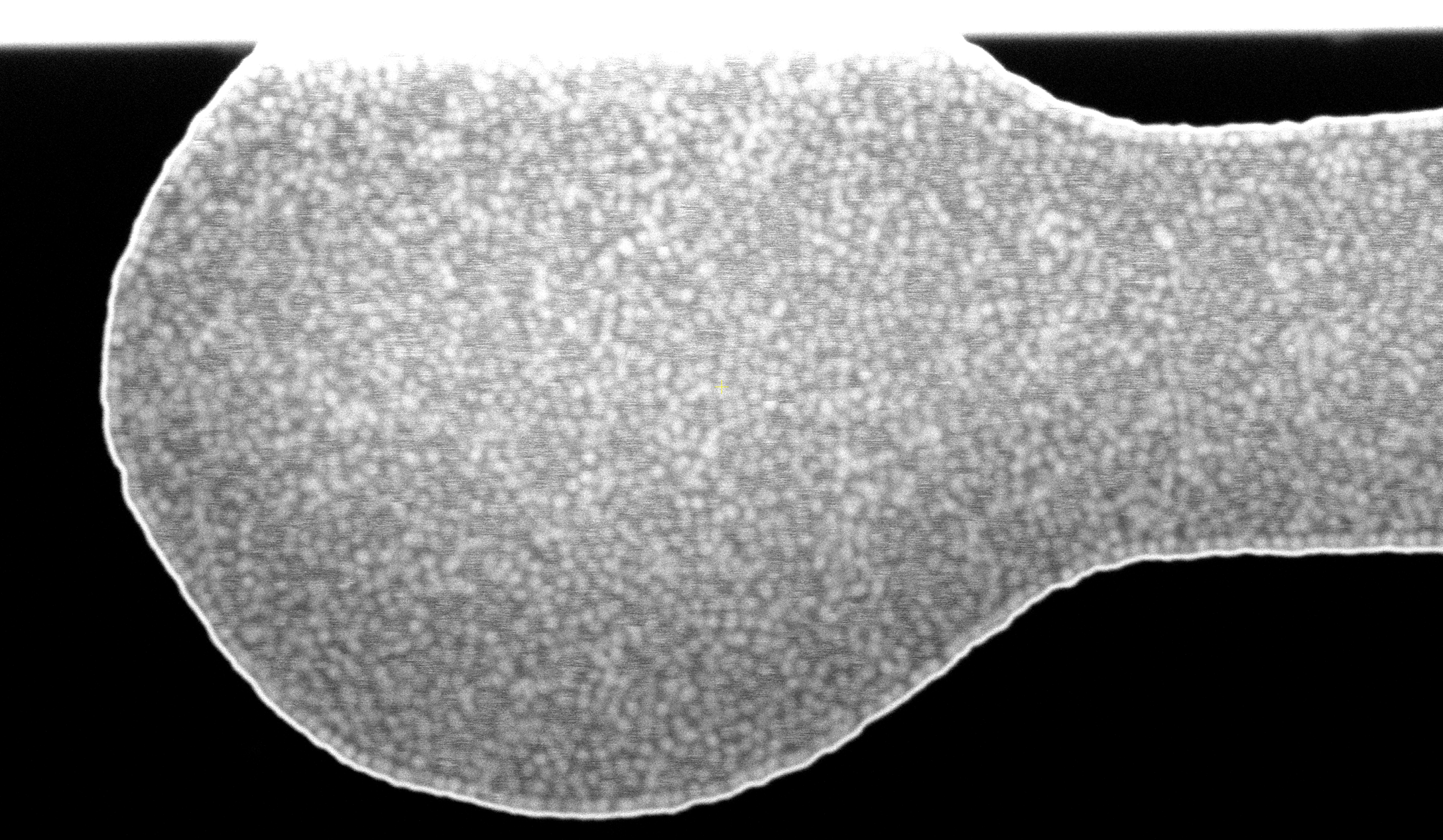
Application note: SEM characterization of SiO2 nano particles in liquid
SEM imaging of 50 nm SiO2 nanoparticles in a liquid environment using our advanced, super easy-to-use liquid cells and our static SEM holder. The results are outstanding, offering great resolution, reproducible imaging, and the potential for high-throughput characterization using SEM.

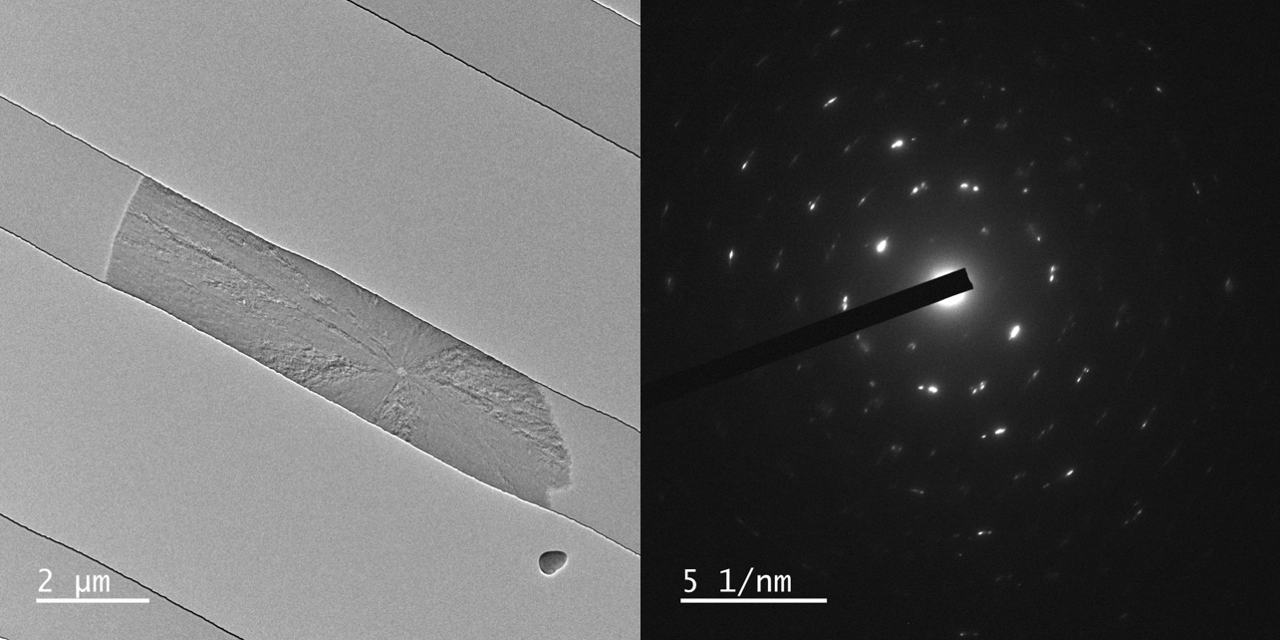
Ferritin in trap chips
Insight Chips has developed the first-ever Nano Channel Trap Chips for TEM, enabling fast, easy, and reliable trapping of nanoparticles in liquid.

PATENTED: Temperature controllled liquid-cell experiment: CaCO3 precipitation captured with atomic resolution
Using Insight Chips' patented solution for liquid-cell heating, precipitation of CaCO3 was captured with atomic resolution by heating up a supersatured solution to 80 degrees C.
Insight Chips' first webinar: Introduction to the Nano Channel Chip and trapping nanoparticles!
Webinar by CEO Emil Jensen and Development Engineer Niccoló Bottauscio

Atomic resolution in liquid-cell TEM!
A compilation of various samples that have yielded atomic resolution images and videos in our Nano Channel Chips.

Liquid-cell Imaging of Gold Nanorods Undergoing Galvanic Replacement by Palladium
Visit with Dr. Parivash Moradifar and Amy McKeown-Green from the Jennifer Dionne Lab at Stanford University yielded interesting STEM images of before and after galvanic replacement of palladium on gold nano rods in liquid.

What is that? Looking for lipid nanoparticles in liquid
While looking for lipid nanoparticles in liquid, interesting things happen...

✨ Wild nucleation in liquid-cell TEM ✨
In a week long demo with Gatan Inc., many interesting results were gathered, one of them out-of-control gold nucleation
.png)
Self-assembly in Nano Channel Chips with Traps Observed in Liquid-cell TEM
A while ago, we introduced our new Nano Channel >Trap< Chips, designed to simplify trapping particles precisely where we want them inside our Nano Channel Chips.

Precise flow control of nano particle solution in Nano Channel Chips using hydrostatic pressure
In this blog we show how particles can be controlled inside the Nano Channel Chips simply by manually lifting the inlet and outlet reserviours up and down, creating a very small change in hydrostatic pressure across the nano channels.

Thickness of the liquid sample
The thickness of the liquid sample is crucial for obtaining a good resolution TEM image, and so is having the right thickness of the Nano Channels.

EELS and 4D-STEM in Liquids: Straightforward with Insight Chips!
With Insight Chips’ Nano Channel Chips, preparation is a matter of minutes. Our innovative design ensures fixed, precisely determined liquid thickness.

Low-Dose Imaging of Nanoparticle Chains in Liquid
Imaging nanoparticle dynamics in liquids AT VERY LOW DOSE RATE.

Useful tool for storing chips wet, or imaging up to 5 chips at once in SEM or optical microscope!
There can be several reasons to store chips wet for a longer time, before inserting them (again) into the TEM holder...

Growing MOFs with Ferritin proteins incorporated inside the Nano Channel Chips!
ZIF-8 MOF grown directly in the Nano Channel Chips - as a bonus, the crystals are filled with ferritin proteins!

Palladium dissolution!
Palladium dissolution observed in the Nano Channel Chips - by researchers at Seoul National Univeristy

Imaging and characterization of nano-sized biological entities
Imaging and characterization of nano-sized biological entities, such as viruses and protein structures, are simplified using the Nano Channel Chip produced by Insight Chips. No freezing, staining, or modification of the samples is needed.

Q-beta protein encapsulating viral RNA
The Q-beta protein sample was introduced into nano channels of the Nano Channel Chip measuring 240 nm in thickness, complemented by 40 nm SiN membranes.